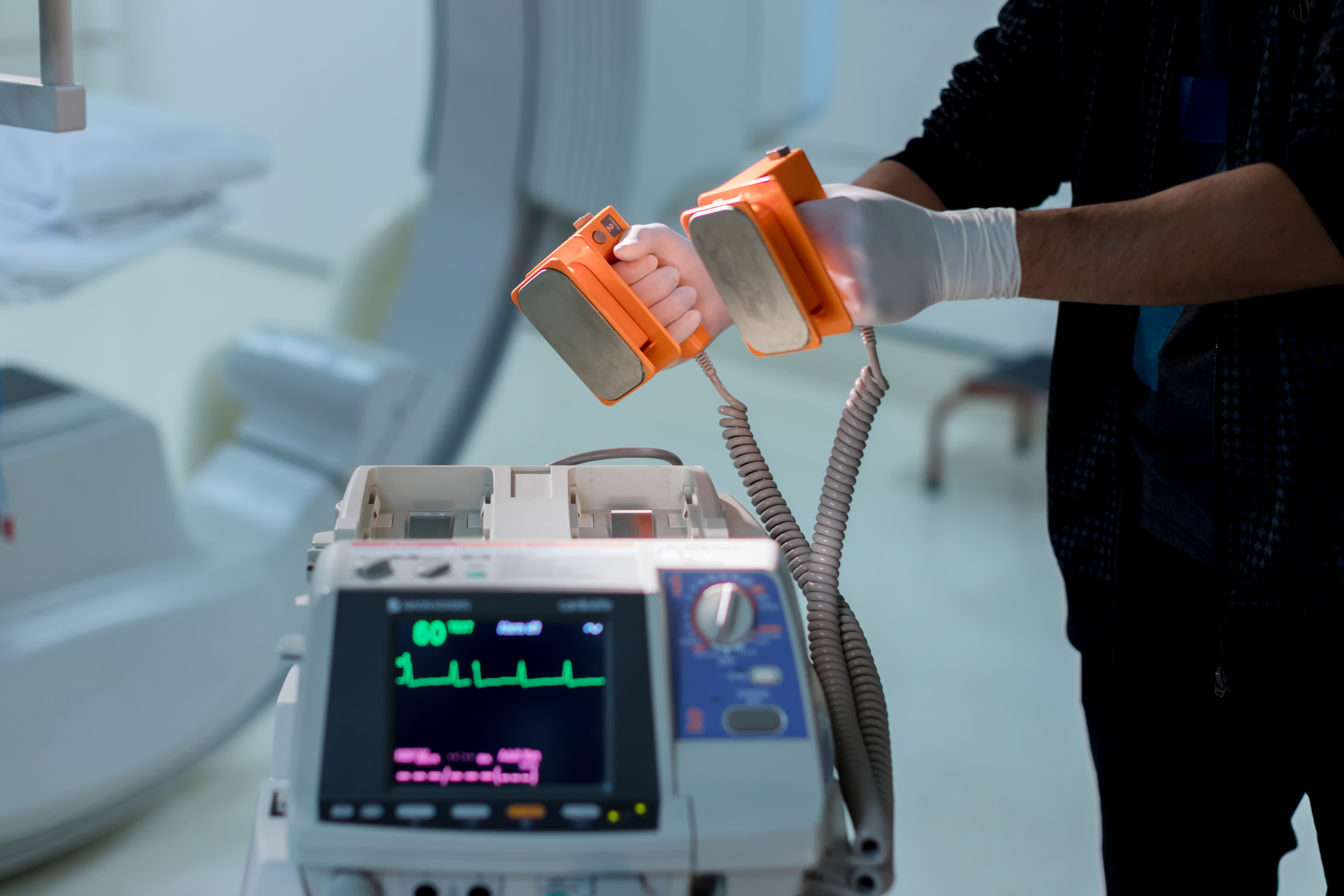
The FDA will now ensure all Automated External Defibrillators in use currently and all future AED devices are reliable life-saving devices. Premarket approval is now required by the FDA for manufacturers of AEDs
In the past, many AEDs were manufactured using inadequate components, insufficient validation of the manufacturing processes along with design issues. In 2013, the FDA alerted users of certain models of AEDs manufactured between 2005 and 2012 that their AED may not deliver a shock to the patient in an emergency situation. Faulty AEDs can result in patient injury or prevent patient rescue.
Check your AED
Organizations and individuals that own an AED system are recommended to check the table here to see if your AED is FDA-approved. If you aren’t sure, you should contact the manufacturer. It is recommended that you continue to hold your AED available for use until you obtain an FDA- approved AED. Any problems related to AEDs should be reported to MedWatch.
Replace Your AED
Are you in need of FDA-approved Defibrillators? CME has dozens of defibrillators including the Zoll R-Series, R-Series Plus, and R-Series ALS. Zoll offers many configurations to suit all provider’s needs. Contact your account manager for more information or call us at 1-800-338-2372. You can also find Zoll defibrillators on our website.
About CME: CME Corp is a full-service healthcare equipment and turn-key logistics company providing personalized support and service. With service centers nationwide, CME offers more than 2 million medical products from a total of over 2,000 manufacturers. CME is a healthcare system's complete equipment solution by providing product selection, sales, warehousing, assembly, staging, direct-to-site delivery, installation, and biomedical services for all its equipment.
About Zoll: ZOLL, an Asahi Kasei company, develops and markets medical devices and software solutions that help advance emergency care and save lives, while increasing clinical and operational efficiencies. With products for defibrillation and cardiac monitoring, circulation enhancement and CPR feedback, supersaturated oxygen therapy, data management, ventilation, therapeutic temperature management, and sleep apnea diagnosis and treatment, ZOLL provides a comprehensive set of technologies that help clinicians, EMS and fire professionals, as well as lay rescuers, improve patient outcomes in critical cardiopulmonary conditions.